Applications
Dot-it-Spot-it® Total Protein Assay
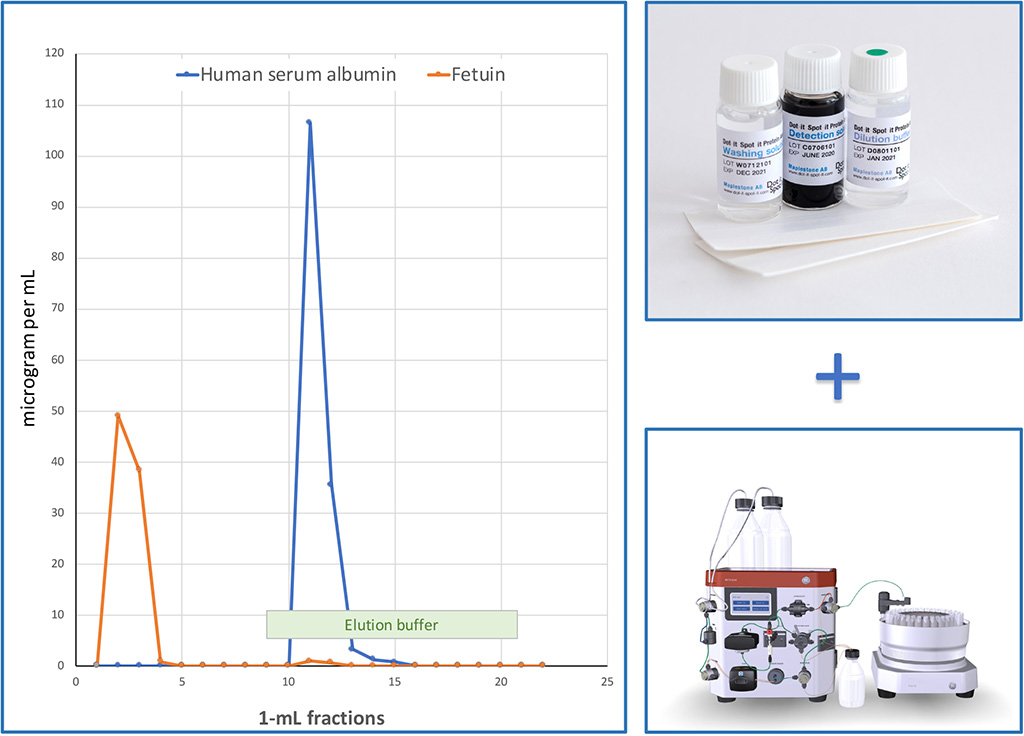
Dot-it-Spot-it is useful for CHROMATOGRAPHY detection
Dot-it-Spot-it test for control of HSA depletion column
Depleted Flow-through fractions:
97 µg Fetuin and < 1.5 µg HSA.
From these valuable 1 mL fractions you need only a few µL for testing.
Eluted fractions:
162 µg HSA and 1.7 µg Fetuin
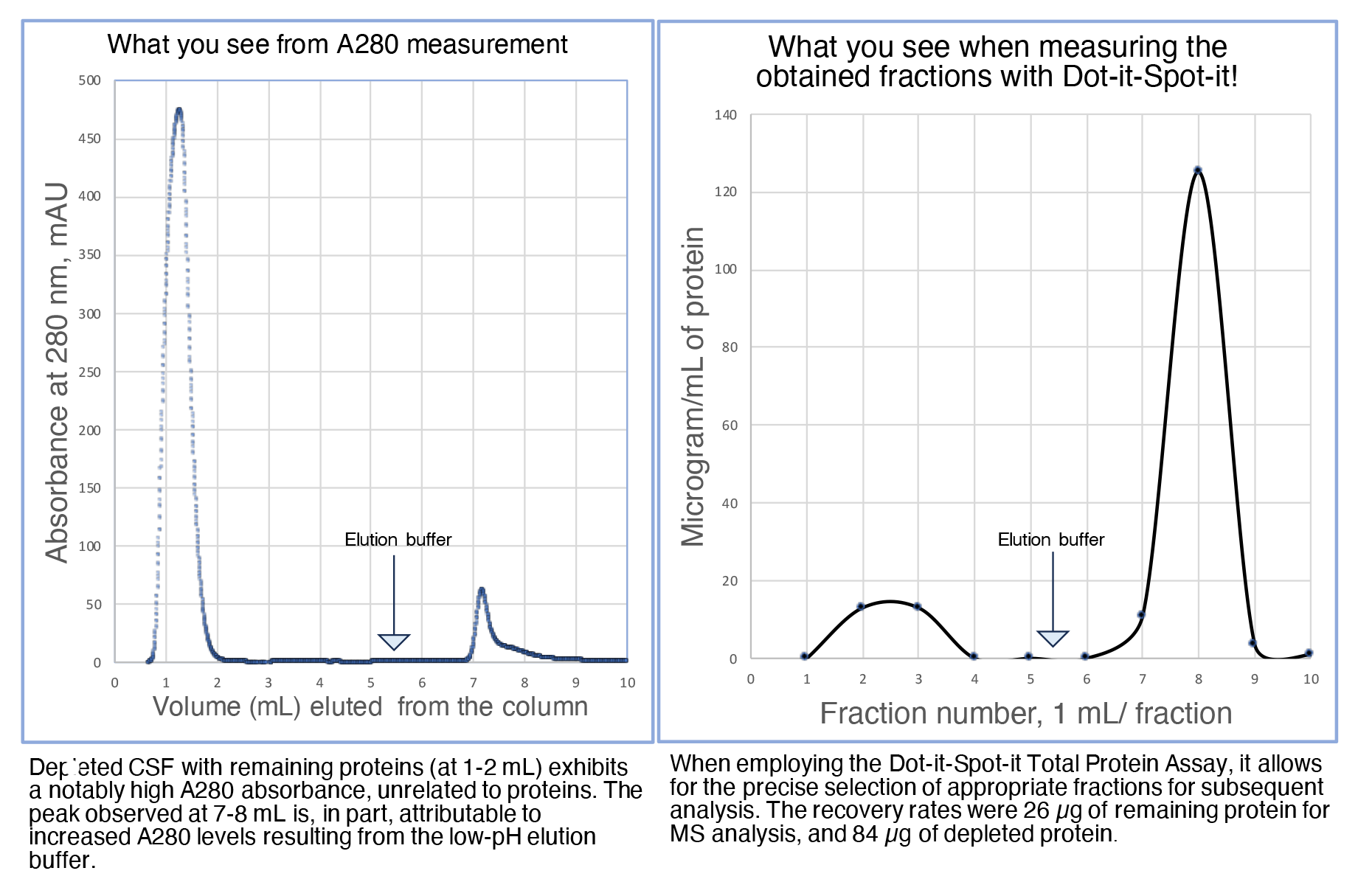
Are you engaged in chromatography of biological fluids with low protein concentrations?
In such cases, it is crucial to supplement the A280 nm detection during the run with the assessment of total protein levels in the fractions.
Take out a few microliters of your fractions and measure down to 0.2 microgram/mL with the Dot-it-Spot-it Total Protein Assay.
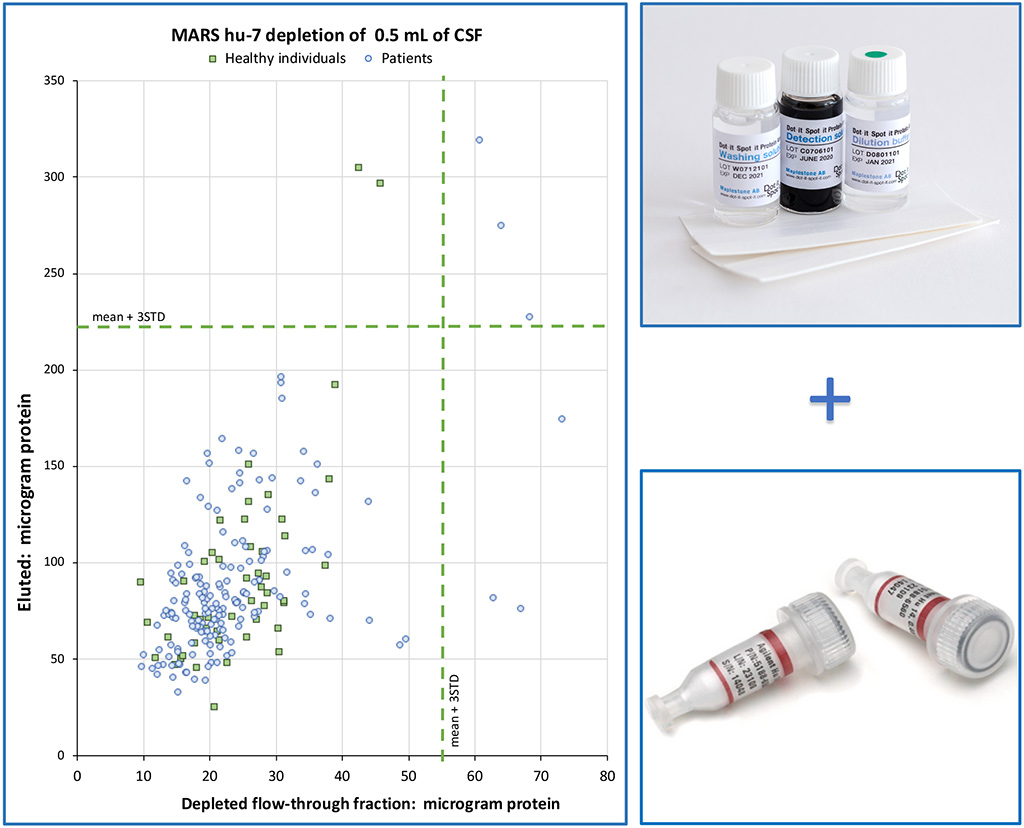
Depleting selected proteins from 0.5 mL of cerebrospinal fluid (CSF).
Utilizing affinity chromatography with a column containing antibodies targeting seven high-concentration proteins present in CSF.
The depleted CSF proteins were subsequently analysed using mass spectrometry (MS). When interpreting the results, it is prudent to include information about the quantity of protein applied to MS,
Dot-it-Spot-it is useful for PRE-TEST control
Depletion of cerebrospinal fluid (CSF) as a pre-step to MS analysis
CSF – check for high protein levels, it may be due to traces of blood proteins.
Follow your proteins in every pre-step.
With Dot-it-Spot-it assay you don’t waste material.
With Dot-it-Spot-it assay you can easily run several hundreds of samples in short time.